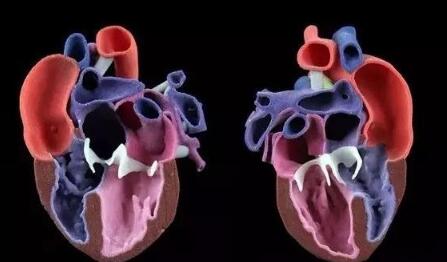
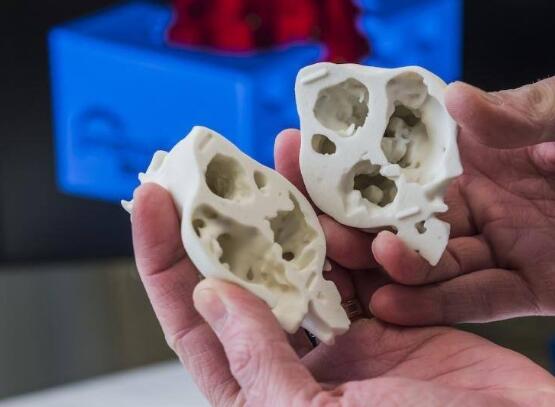
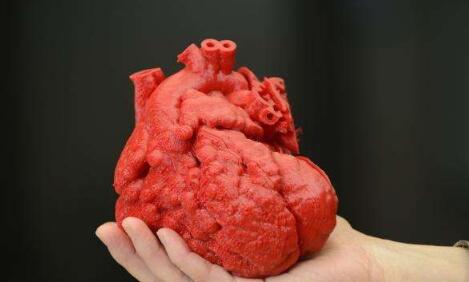
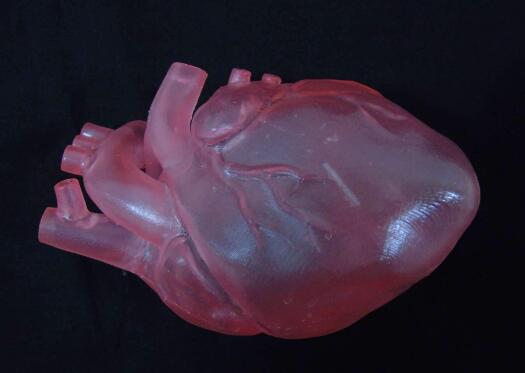
In the early 20th century, the global cardiovascular mortality accounted for less than 10% of the total mortality, but by the early 21st century, cardiovascular mortality had increased significantly, accounting for nearly 50% of the total mortality in developed countries and 25% in developing countries . How to reduce the mortality of cardiovascular diseases is the focus of researchers. Recently, 3D printing technology has gained extensive attention in the field of tissue engineering, providing new ideas for the development of tissue engineering. Compared with traditional tissue engineering, 3D printing technology has the advantages of high precision, fast construction speed, can be manufactured on demand to meet the needs of personalized medical treatment, and low rejection. At present, 3D printing technology has been successfully used in experimental research and has achieved some results. This article mainly describes the research status and progress of 3D printing technology in cardiovascular tissue engineering (myocardial tissue, heart valve, coronary artery). Myocardial tissue When the myocardial tissue is damaged, the myocardium cannot effectively contract and shrink and the scar tissue forms, which eventually leads to ischemic necrosis and death of the patient. The traditional tissue engineering method is cell therapy, which involves repairing cells by implanting cells in the damaged myocardium. But the problem with this method is whether the implanted cells can survive. 3D printing technology can solve this problem. Gaetani et al. Used 3D bioprinting technology to print the three-dimensional structure containing cardiac-derived cardiomyocyte progenitor cells and sodium alginate. After 7 days of cultivation, the survival rates of the two cells were 92% and 89%, respectively, and hCMPCs retained the heart lineage. . In addition, the 3D culture environment increased the expression of transcription factors and protein genes. At the same time, the artificial basement membrane invasion experiment confirmed that progenitor cells can migrate from the alginate matrix, indicating that when this structure is fused with the myocardium, the progenitor cells can migrate to the damaged site of the myocardium. repair. Gaebel et al. Used laser bioprinting technology to print a polyester polyurethane urea heart patch containing human umbilical vein endothelial cells and human bone marrow mesenchymal stem cells, and then implanted the patch into the myocardial infarction area of ​​rats. Examination of the left heart catheter after 8 weeks revealed that blood vessel formation in myocardial infarction area increased significantly and myocardial function was improved. This is a major breakthrough in the study of myocardial function recovery after myocardial infarction. Pati and others use novel extracellular matrix biological inks for 3D bioprinting. The printed three-dimensional structure can provide an ideal microenvironment for cell growth. They then encapsulated the myoblasts of mice into the acellular heart matrix bio-ink structure. After 4 days of cultivation, they found that the myoblasts continued to express myocardium-specific genes for at least 14 days. In addition, the acellular heart matrix bio-ink structure causes the expression of myocardial myosin heavy chain to be higher than that of collagen structure. At present, although the porous three-dimensional structure constructed by the 3D bioprinting technology has made some breakthroughs in maintaining cell viability, whether it can functionally regenerate damaged myocardial tissue and restore systole to maintain blood pumping function requires further experiments in the future the study. Heart valve At present, patients with heart valve disease have only two options when undergoing heart valve replacement surgery: (1) Use artificial mechanical heart valves; (2) Use biological heart valves. Compared with biological valves, artificial mechanical valves have the advantages of strong mechanical properties and long service life, but long-term anticoagulation treatment is required. Biological valves do not require anticoagulation therapy, but they often cause calcified or non-calcified structural damage and have a shorter service life than artificial mechanical valves. Therefore, there are some problems of compatibility, durability and growth potential of artificial mechanical valves and biological valves. The three major elements for constructing tissue-engineered heart valves are seed cells, scaffold materials, and cell planting. As a living organ, tissue engineering heart valve avoids the shortcomings and deficiencies of mechanical valve and biological valve after implantation, and its growth, repair and reconstruction ability is very similar to that of normal human valve. Recently, the use of 3D bioprinting technology to generate valves has become a hot spot in the field of valve research. Duan et al. Used bioprinting technology to print aortic valves with complex anatomical structures, which consisted of sodium alginate or gelatin containing aortic sinus smooth muscle cells and aortic valve leaf interstitial cells. After 7 days of cultivation, the survival rates of the two types of cells were (8.14.4 ± 3.4)% and (833.2 ± 4.0)%, and both cells expressed α-smooth muscle actin And vimentin. The results of these studies indicate that it is feasible to print the aortic valve using 3D bioprinting technology, and it can be used in the clinic eventually. Subsequently, Duan et al. Used bioprinting technology to print the tricuspid valve again, which was composed of mixed hydrogel and aortic interstitial cells. The study found that increasing the concentration of mixed hydrogel will result in lower hardness and higher viscosity , Promote cell proliferation and better maintain the fibroblast phenotype of hAVICs. The hAVICs encapsulated in bioprinted heart valves remain highly viable and change the initial matrix through the deposition of collagen and mucopolysaccharides. The results of these studies will accelerate the progress of new valve replacement research and lay a solid foundation for future clinical applications. Although tissue-engineered heart valves have many advantages such as low immunogenicity, no cytotoxicity, better bionicity, durability and mechanical strength, there are still many problems that need to be solved, such as the selection of seed cells and the Rapid expansion, increase cell adhesion, optimal number and proportion of seed cells, simulate in vivo stress and microenvironment to construct tissue engineering heart valves, etc. Coronary artery Coronary artery disease is a common disease with the highest incidence in Europe and the United States and the leading cause of death. The symptoms of coronary artery disease can be controlled by lifestyle changes, medical treatment, coronary angioplasty or coronary artery bypass grafting. For complicated multi-vessel coronary artery disease, coronary artery bypass grafting is a routine clinical treatment. However, many patients have vascular stenosis after the operation, which leads to the failure of the operation, and about 30% of the patients cannot perform surgical treatment due to the lack of proper autologous blood vessels. Vascular tissue engineering provides new ideas for coronary artery bypass grafting. Ideal tissue-engineered blood vessels should be endothelialized, do not form thrombus, and have biomechanical characteristics comparable to natural blood vessels. At present, 3D printing technology is of great research value in generating biomimetic blood vessels, because it can not only generate specific scaffolds based on specific patients using synthetic biomaterials, but also connect endothelial cells, fibroblasts or mesenchymal stem cells Capacitive materials are printed together. Wu et al. Demonstrated the all-round printing 3D bionic microvascular network. They deposited volatile ink into the cured gel reservoir to form a layered, branched network, and then removed the ink by liquefaction to obtain the desired microvascular network in the gel reservoir. However, this research did not integrate the cells into the constructed microvascular network. Miller et al. Used a 3D printing technique to print a cylindrical blood vessel network consisting of carbohydrate glass and endothelial cells on both sides of the cylindrical channel. After 9 days of culture, the cross-sectional imaging showed that the blood vessel cavity was surrounded by endothelial cells, and the endothelial cells formed single or multicellular buds. This study confirmed the three key components of vascularized solid tissue: vascular lumen, endothelial cells, stroma, and cells residing in the interstitial region. Li et al. Used a double-head nozzle printing technology to print the hydrogel vascular network. They formed a hydrogel containing adipose-derived mesenchymal stem cells into a network frame, and placed a hydrogel containing liver cells around the network frame. After assembly, the structure was stable and ADSCs were induced to differentiate into similar endothelial cells. Moreover, the ASCs on the periphery of the vascular network have been confirmed to have some characteristics similar to endothelial cells. These research results have confirmed that the use of 3D printing technology can generate tubular blood vessels. However, printing the ideal artificial coronary artery through 3D printing technology still faces the following three challenges: (1) There are three layers of autologous blood vessels, and each layer has different cell composition, stiffness, and function. It is difficult for the printed blood vessels to simulate the structure and function of each layer. (2) Almost all tissue-engineered blood vessels printed by 3D printing technology are microscale. (3) The cells present in the artificial coronary artery must survive the uninterrupted supply of oxygen and other nutrients. These issues all need further study in the future. The application of 3D printing technology in cardiovascular tissue engineering has been widely recognized. But the current research is in the initial stage, and there are still many problems to be solved. First, due to the inherent properties of hydrogels, and the hydrogels used for printing must maintain a low viscosity to prevent nozzle clogging, the printed structure does not have sufficient mechanical strength to maintain its shape and withstand external pressure. Therefore, choosing the appropriate hydrogel material (mechanical properties, diffusion coefficient, biocompatibility, compatibility) is necessary for the long-term success of bioprinting technology. Secondly, the complex structures of tissues and organs printed by 3D printing technology including multiple cell types do not have high resolution and need to be further improved. Finally, the vascularization of engineered tissue is a key issue. Many studies have shown that neovascularization can be formed in a two-dimensional structure, but it is difficult to form neovascularization in a three-dimensional structure. Although 3D printing technology has made some progress in tissue vascularization, the manufacture of fully vascularized tissue structures for clinical application still requires further research. With the continuous development of 3D printing technology and the in-depth study of cardiovascular tissue engineering, artificial cardiovascular tissue is expected to be widely used in the treatment of cardiovascular diseases, laying a solid foundation for humans to overcome cardiovascular diseases. Editor in charge: Liu Yang Double 4-layer Shelf,Tv Stand,Wine Cabinet Jiangmen Lihua Import & Export Trading Co., Ltd. Jiangmen Lihua Furniture., Ltd. , https://www.jmlihua.com